The Food and Drug Administration (FDA) is proposing to amend their regulations governing the format of the National Drug Code (NDC). The NDC is a standard for uniquely identifying drug products marketed in the United States. The current standard has several acceptable formats. If the proposal is finalized, it will standardize the format of all NDCs.
What is the NDC?
The National Drug Code is a unique, three-segment identifier that the FDA assigns to each drug on the U.S. market. It’s a universal product identifier that appears on all prescription and over-the-counter medication packages and inserts.
Current formats:
10-digit identifier
The FDA’s standard NDC is a 10-digit numerical identifier that includes a labeler code, product code, and package code.
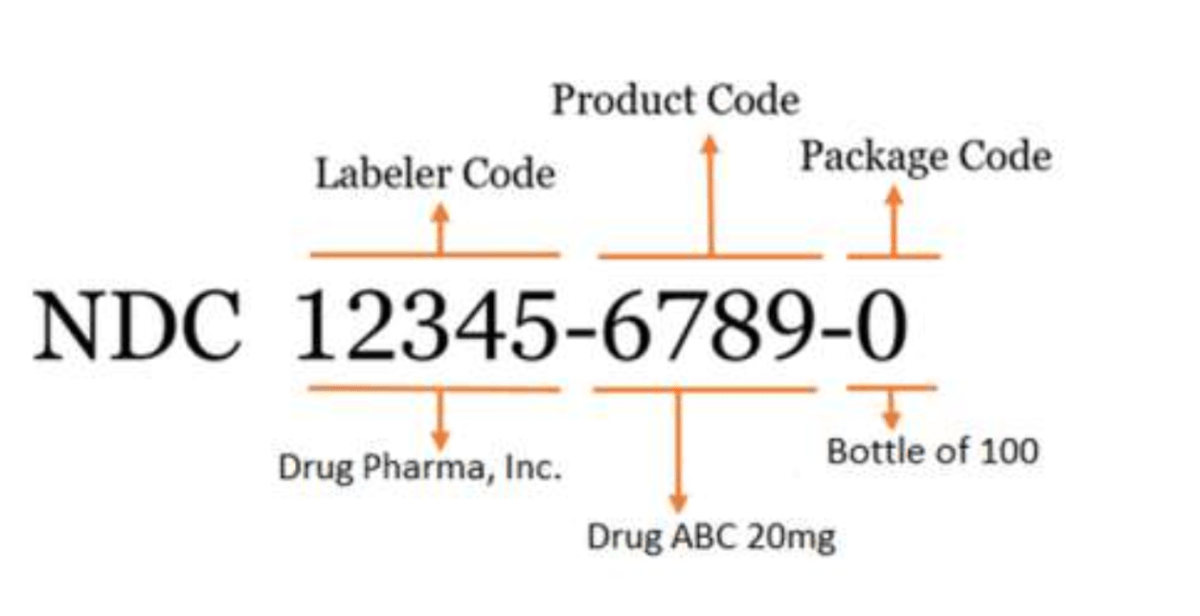
There are 3 FDA-assigned formats for the standard NDC:
- 4-4-2
- 5-3-2
- 5-4-1
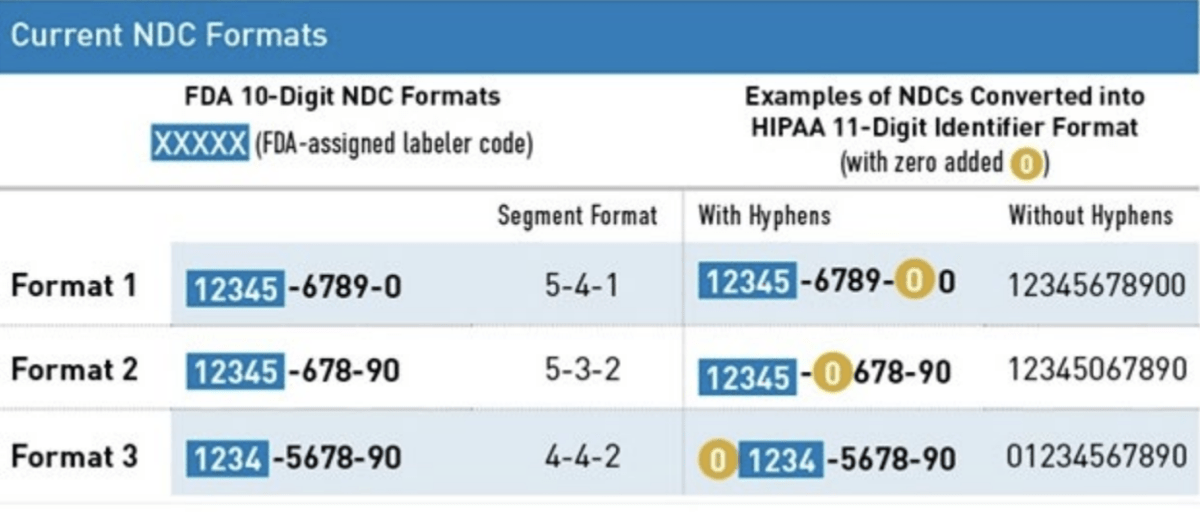
HIPAA Format
The Health Insurance Portability and Accountability Act (HIPAA) adopted a uniform 11-digit NDC format that must be used when a HIPAA-covered transaction includes an NDC. This 11-digit format is standardized into a 5-4-2 format and created by adding a leading zero to either the labeler, product, or package code.
Upcoming 6-Digit Format
The FDA will run out of 5-digit labeler codes in 10-15 years. Per FDA regulations (21 CFR 207.33), once FDA runs out of 5-digit labeler codes, it will start assigning 6-digit labeler codes. Without this proposed change, there would be five NDC formats, 3 in 10- digits and 2 in 11-digits. There may be confusion between an FDA-assigned 11 digit NDC and a HIPPA converted 11-digit NDC.
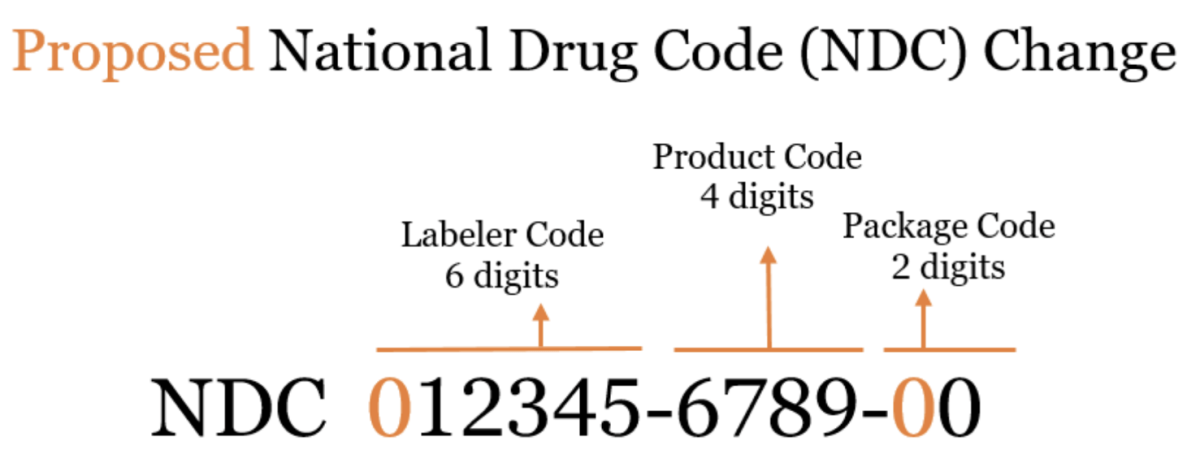
The Proposed Rule
The proposed rule modifies existing regulations to establish a uniform, 12-digit format that can accommodate longer NDCs once the FDA begins issuing 6-digit labeler codes.
The change will impact a variety of industries and stakeholders including:
- Human and animal drug manufacturers and distributors
- Drug importers
- Federal agencies using the NDC
- Drug databanks
- Pharmacies
- Hospitals, clinics, labs, healthcare practitioners
- Nursing care facilities
- Electronic health record vendors
- State and local governments
- Various supply chain stakeholders
The rule change would standardize the NDC format across all sectors and minimize confusion and medication errors.
If the rule is adopted, it is expected to have a five-year delayed effective date in addition to a three-year transition period to allow for preparation and implementation. The proposed changes, whether or not the rule is finalized, signal a significant shift in NDC formatting to streamline identification and reduce confusion.
Diaz Trade Law will continue to monitor developments concerning this proposed rule. We provide guidance on a variety of FDA matters including food, cosmetics, drugs, alcohol, medical devices, and more.
Learn more about FDA compliance: